63+ calculate the amount of heat required to raise the temperature
Q mCΔT where Q is the amount of heat. Amount of heat Mass Heat.

Pdf Analysis Of The Subsurface Urban Heat Island In Oberhausen Germany
Determine the change in temperature of the system.

. Youll get a detailed solution from a subject matter expert that helps you learn core concepts. Web Heat capacity is the amount of heat required to change the temperature of a given amount of matter by 1C. Web To calculate this well use the specific heat formula.
As you know a substances specific heat tells you how much heat is needed in order to increase the temperature of 1 g of. Web QA Heat Required to Raise Temperature of Materials During Heat-Up Wh QB Heat Required to Raise Temperature of Materials Processed in Working. Determine the mass and specific heat of the system.
Web A British thermal unit BTU is defined as the amount of heat required to raise the temperature of 1 pound of water by 1 degree Fahrenheit. Web Calculate the amount of heat required to raise the temperature of an 64 g sample of water from 32 C to 69 C. Web The amount of heat required to raise the temperature of water sample can be calculated using the formula.
Web The Specific Heat c of water is 4186 joulegram C. Web Calculate the heat required to raise the temperature of 286 g of sodium from 337 C to 738 C. Web This problem has been solved.
Web Take a look at the specific heat of water. There are two sig figs in the given. Answer 9000 J alternatives 90 x 103 J Question 2 900.
Q m c ΔT where q is heat energy m is mass c is specific heat constant for the particular material or. 141x103 j The lowest. Web Calculate the amount of heat required to raise the temperature of a 63 g g sample of water from 35 C to 69 C Calculate the amount of heat required to heat a 49 kg.
M50 Kilograms c4200 J kg C J k g C Step 2. So Q 76g 4186 joulegram C 62C - 31C or Q 9862216 joules. Web The specific heat capacity also called specific heat represented by the symbol c text c c start text c end text or C text C C start text C end text is how much energy is needed.
ΔT 7060 Δ. Calculate the amount of heat required to. The heat capacity of 1 gram of a substance is called its.
Web 1 Calculate the amount of heat required to raise the temperature of a 65-g sample of water from 32 C to 65 C. The specific heat capacity of sodium is 123 Jg C.

Pdf Heat Exchange And Transport In The Environ Ent Prepared For Electric Power Research Institute Cooling Water Discharge Research Project Rp 49 John Edinger Academia Edu

Openstax College Physics Solution Chapter 14 Problem 17 Problems Exercises Openstax College Physics Answers

Concepts Of Physics 2 For Iit Jee Class 12 H C Verma Harish Chandra Verma Bharati Bhawan Dokumen Pub

Which Of The Following Processes Requires The Most Energy Input Given Below Is The Properties Of The Water Propertyvalue Specific Heat 1 0 Cal G O Clatent Heat Of Fusion 80 Cal Glatent Heat Of Vaporization540

Chemistry 103 Principles Of Chemistry Pdf Pdf Chemical Compounds Chemical Bond

Answered Is The Amount Of Energy It Will Absorb Bartleby

Temperature And Phase Changes Answers
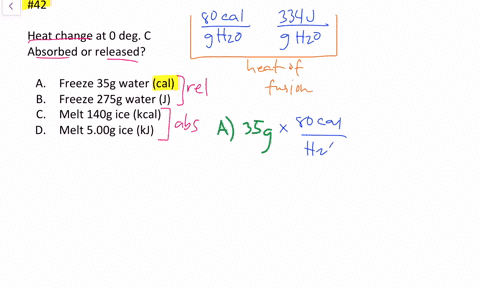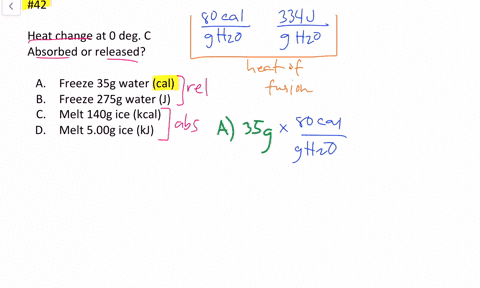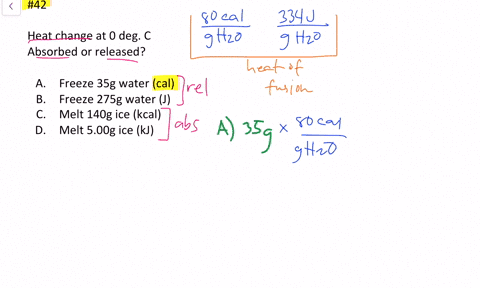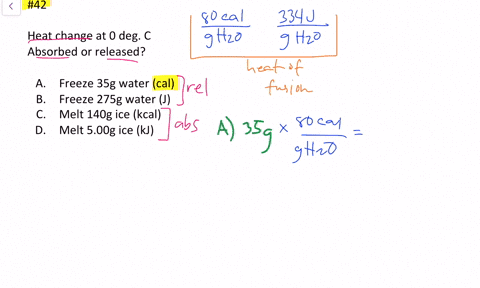
Solved Calculate The Heat Change At 0 C For Each Of The Following Problems Indicate Whether Heat Was Absorbed Or Released A Calories To Melt 65 G Of Ice B Joules To Melt
Calculate The Amount Of Heat Required To Raise The Temperature Of 5 G Of Iron From 25 C To Youtube

Heat Capacity

Specific Heat Calculator

Find The Amount Of Heat Released If 1 Kg Steam At 200 C Is Converted Into 20 C Ice

1 Ap Pdf

Answered 8 A Piece Of Unknown Metal With A Mass Bartleby

Heat Capacity

Specific Heat Mc T Specific Heat The Amount Of Heat Energy A Material Requires To Raise Its Temperature Is A Characteristic That Can Be Used To Identify Ppt Download

Answered 4 What Is The Temperature Change For Bartleby